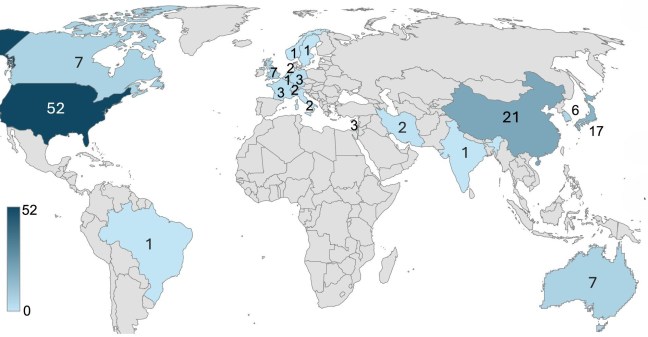
STEM-PD investigators, Gesine Paul and Agnete Kirkeby, alongside researchers from Kyoto and the BlueRock Therapeutics have recently published a book chapter summarising the current ongoing clinical trials using dopamine cell replacement in Parkinson’s and the respective cell sources used in these clinical trials.
In this chapter, the authors discuss cell therapies for Parkinson’s disease (PD) as an exciting and promising treatment option in clinical trials, showing early signs of safety and effectiveness. Although these trials are still in the early phases, they offer valuable lessons to improve future designs and optimize future potential cell products. The authors also describe the challenges that remain, such as the need for immunosuppression.
The authors consider the efforts that are underway to overcome certain challenges, such as the need for immunosuppression by creating hypoimmune universal cell lines that can evade the immune system and improve graft survival, along with better methods for producing and scaling cell products.
This chapter is an interesting read for anyone who is interested in the field of dopamine cell replacement therapy for PD. You can read the fulll chapter blow:
Full article: https://doi.org/10.1016/bs.irmvd.2024.08.004
This chapter was published in International Review of Movement Disorders, Vol 7, Gesine Paul, Asuka Morizane, Agnete Kirkeby, Jun Takahashi, Claire Henchcliffe, Chapter Ten – The future: Stem cells? Current clinical trials using stem cells for dopaminergic cell replacement, Page 191-220, Copyright Elsevier (2024)